Research Interests:
Harmful algal blooms (HABs), Molecular and Cellular Toxicology of Algal Toxins, Marine Mammals, Trophic Transfer, Transcriptional Biomarkers, Structure-Activity Relationships, Nanoparticle Toxicity
Research Projects:
Harmful algal blooms (HABs) are commonly known for their detrimental impacts on aquatic organisms (including marine mammals), human health, and also local economies. Our lab specializes in cellular and molecular toxicology with a strong emphasis on algal toxins and HABs. We employ a variety of cell culture techniques to discern the cytotoxicity and cell signaling effects of algal toxins on in vitro model cellular systems as well as various molecular techniques such as gene expression microarrays, RT-PCR, Western blots, gel electrophoresis, and reporter gene assays. Once a toxin's target or mechanism of action has been identified, we perform biochemical enzyme activity assays to characterize binding affinities and structure-activity relationships (SAR).
Molecular Pharmacology / Toxicology:
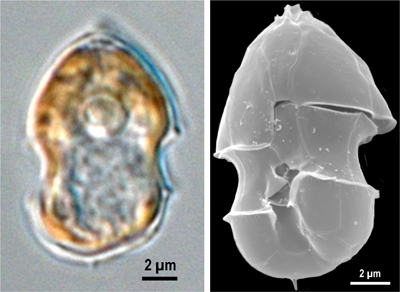
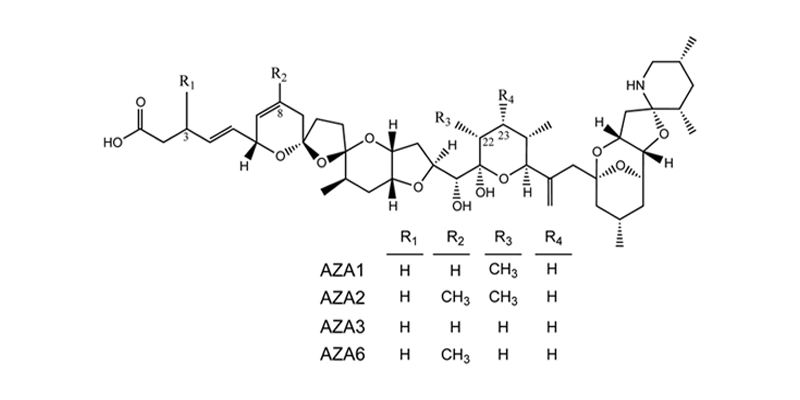
Azaspiracids (AZAs) are highly potent algal toxins produced by the recently identified small dinoflagellate Azadinium spinosum. AZAs have caused human health outbreaks in many European countries and more recently in the US. A. spinosum and/or AZAs have since been found throughout the world in Chile, Japan, Canada, Morocco, Argentina, USA, and New Zealand representing an emerging threat to public safety. Our lab is extensively involved in investigating the mechanism(s) of action of the various AZA analogues. Our investigations have employed in vitro cell models to characterize the potential toxicological impacts of AZA1, AZA2 and AZA3, and the use of DNA microarrays (i.e., gene chips) has yielded valuable insights as to possible biochemical pathways targeted by AZA1. Studies with several mammalian cell types yielded low nanomolar EC50 cytotoxicity values, making the AZAs one of the most cytotoxic algal toxin groups known. In some groundbreaking studies, we have recently identififed AZAs as hERG potassium channel inhibitors.
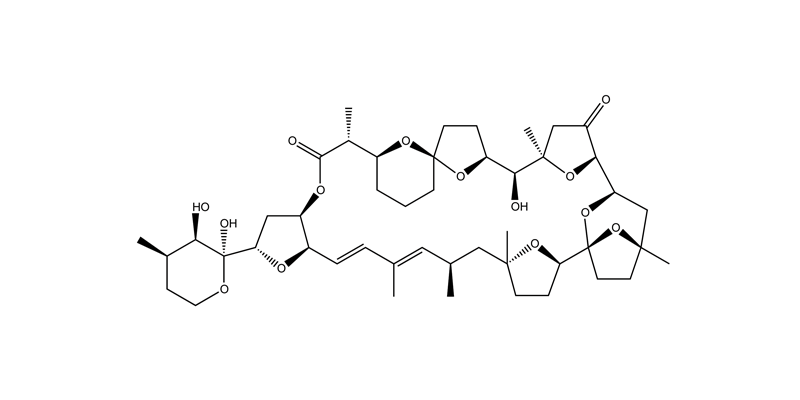
Okadaic Acid (OA) and the closely related dinophysistoxins (DTXs) and) are known ser/thr protein phosphatase (PP) inhibitors produced by toxigenic species of Dinophysis and Prorocentrum. Although they have been extensively studied as tumor promoters with specific inhibitory activity towards PP2a, and to a lesser extent PP1, the current understanding of the OA/DTX-binding site(s) is incomplete. Furthermore, little is known with respect to affinity towards other phosphastases.
Pectenotoxins (PTXs) are marine algal toxins produced by species of Dinophysis known to naturally accumulate in shellfish representing a serious human health concern for seafood consumers from many countries around the world. To date, at least 15 different analogues of PTX have been isolated and structurally characterized. Although monitored and regulated in many European countries, there are no regulatory requirements or standards here in the United States. This did not prove to be a problem until 2009 when PTXs were identified for the first time in US seafood (razor clams, mussels, and oysters). Previous studies have shown that the mechanism of action for the parent PTX analogue is via inhibition of actin polymerization. Actin proteins are a major constituent of the cytoskeletal structure and are essential to many cellular processes such as cytokinesis, cell adhesion, cell movement, and intracellular trafficking and support. Inhibition of actin polymerization can result in cell death. Our lab is involved in characterizing the effects of various PTXs on actin polymerization and depolymerization.
Algal Toxin Trophic Transfer studies:
Gulf of Mexico bottlenose dolphins (Tursiops truncates) are top marine predators and can serve as important sentinels of coastal environmental health. In the Sarasota region, the resident dolphin populations have been observed and/or sampled during health assessment studies for last 40 years. Recent findings have shown that these mammals are exposed to various algal toxins such as brevetoxin and domoic acid on a nearly annual basis. Exposure of these toxins appears to be via primary food items such as pigfish, pinfish, mullet, and scaled sardines. Preliminary health parameters suggest that exposed animals may have an elevated immune response. Gulf of Mexico marine mammals such as bottlenose dolphins and manatees are susceptible to periodic unusual mortality events (UMEs).
We have conducted extensive studies investigating algal toxin exposures (brevetoxin, domoic acid) and toxicokinetics (absorption, distribution, metabolism, and elimination) in stranded bottlenose dolphins along the Florida Panhandle and Central West Florida regions. We have recently undertaken studies investigating the exposure and possible trophic transfer of microcystin algal toxins in Lake Erie fish. Of particular relevance due to human consumption patterns include walleye, bass and perch.
Click here to watch a recent PBS broadcast by our colleagues at University of Tennessee and Bowling Green State University discuss Microcystis issues in a small Ohio lake.
Transcriptional Biomarker development:
Working in conjunction with colleagues at the University of Tennesse and the University of Plymouth (UK), we are currently involved in exposure experiments of microcystins and Microcystis to both zebrafish and channel catfish. These studies are focused on developing transcriptional gene expression biomarkers for use as diagnostic and prognostic indicators of exposure and toxicity. These studies are being performed in conjunction with histopathological analyses. Click here to read about this project in a local newspaper.
Emerging Toxigenic HAB events:
In collaboration with NOAA Charleston, the College of Charleston, and the University of Washington, we are currently involved in documenting the presence and toxicity of domoic acid-producing Pseudo-nitzschia species in the biologically productive Benguela Upwelling Zone along the western coast of Africa.